Hydrogen Bonding Competition Between Water and Hydrogen Sulfide: MW Spectroscopy of Microsolvation of Benzyl Alcohol and Benzyl Mercaptan
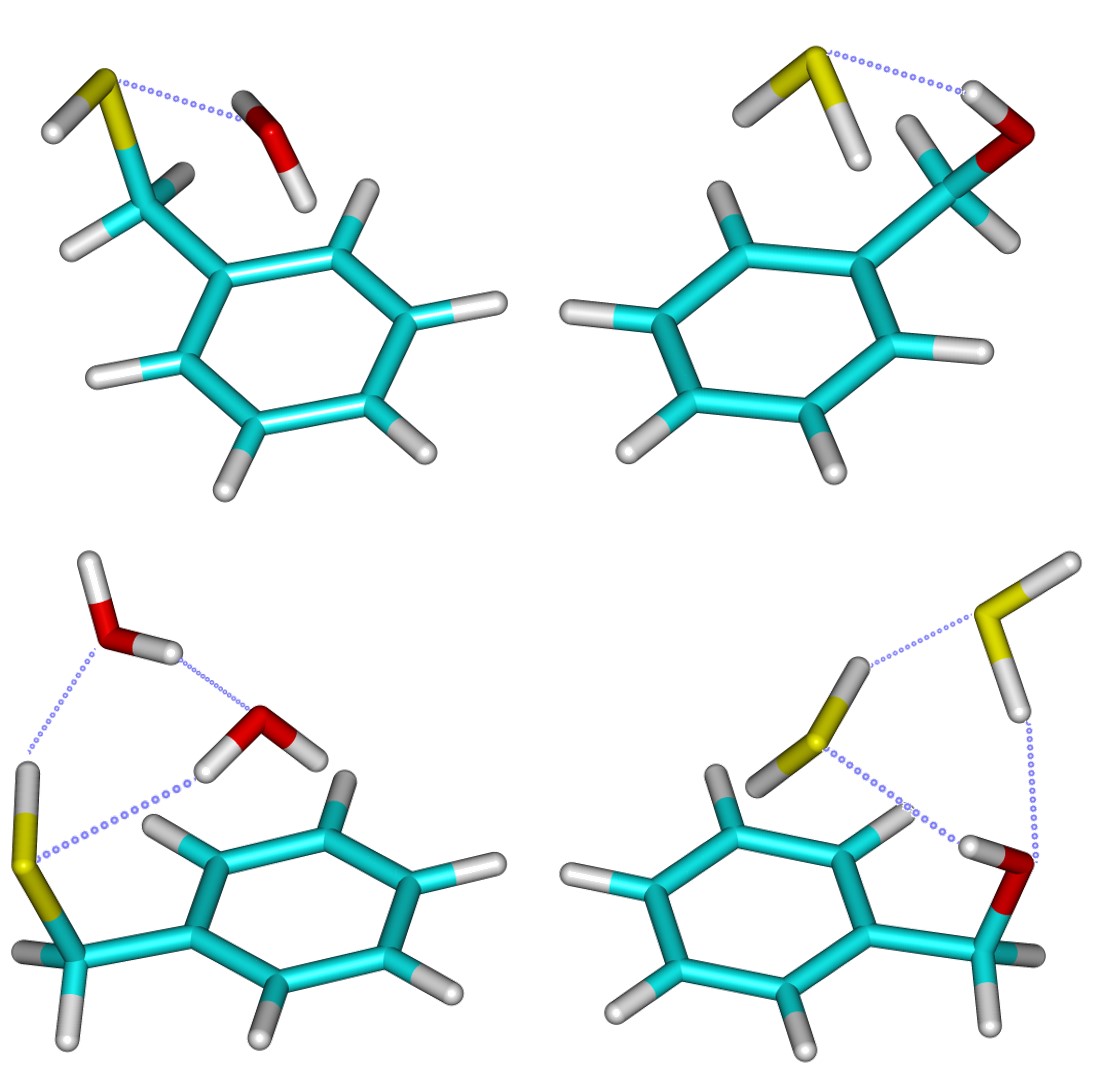
We observed several intermolecular complexes involving H2O or H2S with benzyl alcohol and benzyl mercaptan, analysing the microsolvation and hydrogen bonding competition between the two hydrides. The clusters were generated in a jet-cooled expansion and characterized using chirped-pulse Fourier-transform microwave spectroscopy in the region 2-8 GHz. In the benzyl alcohol monosolvates, the aromatic alcohol acts as a proton donor, in opposition to the benzyl mercaptan dimers where the thiol group acts as a proton acceptor. For the bisolvated trimers, we observed two H2O or H2S molecules binding to benzyl alcohol and two water molecules attaching to benzyl mercaptan, always stabilized by cooperative hydrogen bonding. The clusters additionally involved secondary interactions between the solvents and the π ring system. Detailed information on the noncovalent interactions and molecular structures results from the combination of rotational data and DFT and ab initio molecular orbital calculations.