Accurate Gibbs Energies of Complex Formation
The formation of a molecular complex relies on the Gibbs energy of formation, ΔG, which is often difficult to obtain accurately from pure experimental or theoretical methods. A hydrogen bound bimolecular complex consists of a hydrogen bond donor and acceptor unit. The OH-stretching fundamental transition of the hydrogen bond donor is typically redshifted and its infrared intensity enhanced upon complexation.[1] This facilitates detection of weak complexes even though the equilibrium is strongly shifted towards the monomers at room temperature. The ratio of a measured and calculated intensity of a vibrational band is proportional to the complex abundance, which with known monomer pressures gives the equilibrium constant.[2,3] We have developed a reduced dimensionality variational local mode model, which includes both high- and low-frequency vibrations, and we use this model to calculated absolute transition intensities.
We have used this combined experimental and theoretical approach for a number of bimolecular complexes and recently extended it to hydrated complexes. The spectrum of the water-trimethylamine complex is shown in the figure.[3] The equilibrium constant obtained from the observed five different bands should be equivalent, and the detection of multiple bands therefore allows us to validate the accuracy of our combined experimental and theoretical approach. We believe the accuracy of ΔG for the water-amine complexes is better than 1 kJ/mol.[3]
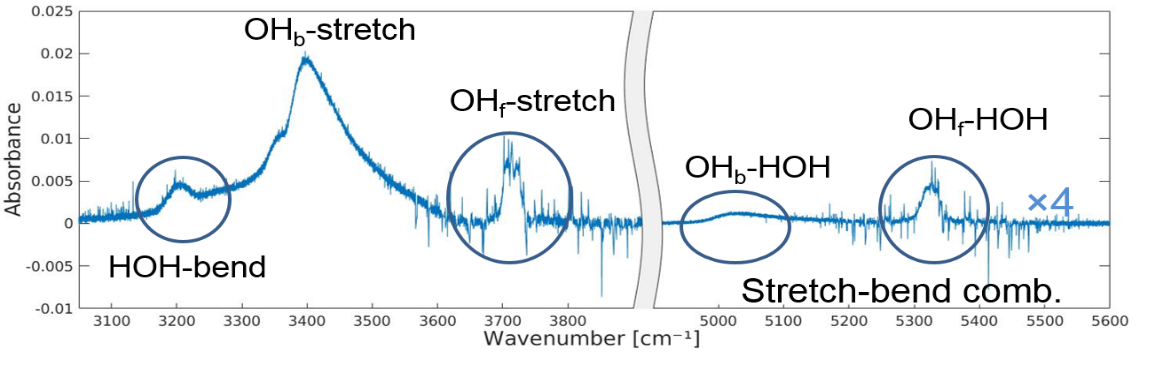
The water dimer, H2O-H2O, is an atmospherically important hydrogen bound complex. Most spectroscopic studies on water dimer are performed under non-equilibrium cold conditions, which favor complex formation; however, in an atmospheric context information at ambient temperature is important. As a first step, we have recently developed a variational local mode model for water dimer that include all six intramolecular modes and the three most important intermolecular modes. The calculated fundamental vibrational transition frequencies and relative intensities agree well with results from jet-cooled experiments.
[1] Arunan, Elangannan, et al. Pure Appl. Chem., 2011, 83, 1619-1636.
[2] Anne S. Hansen, Emil Vogt, H.G. Kjaergaard, Int. Rev. Phys. Chem., 2019, 38, 115-148.
[3] Alexander Kjaersgaard, Emil Vogt, Anne S. Hansen, H.G. Kjaergaard, J Phys Chem A, 2020, 124, 7113-7122.