The effects of two inequivalent internal rotations in acetylmethylthiophenes explored by microwave spectroscopy
The microwave spectra of three isomers of acetylmethylthiophene were recorded using a pulsed molecular jet Fourier transform microwave spectrometer operating in the frequency range from 2.0 to 26.5 GHz. Quantum chemical calculations predicted two stable conformers for all isomers, possessing either a syn- or an anti-orientation of the acetyl group. Both conformers were experimentally observed for 2-acetyl-4-methylthiophene (2A4MT, molecule (2) in Figure 1), while the spectra of the other two isomers showed exclusively transitions belonging to one conformer; anti in the case of 2-acetyl-3-methylthiophene (2A3MT, molecule (1) in Figure 1) and syn in the case of 2-acetyl-5-methylthiophene (2A5MT, molecule (3) in Figure 1).
Due to the internal rotation of the acetyl methyl and the ring methyl groups all rotational transitions split into five torsional species. These splittings could be resolved and analyzed for all assigned conformers, yielding the torsional barriers given in Figure 1. The impact of the substitution position where the methyl internal rotor attached to the aromatic thiophene ring and the relatively constant torsional barrier around 300 cm-1 of the acetyl methyl rotor will be discussed.
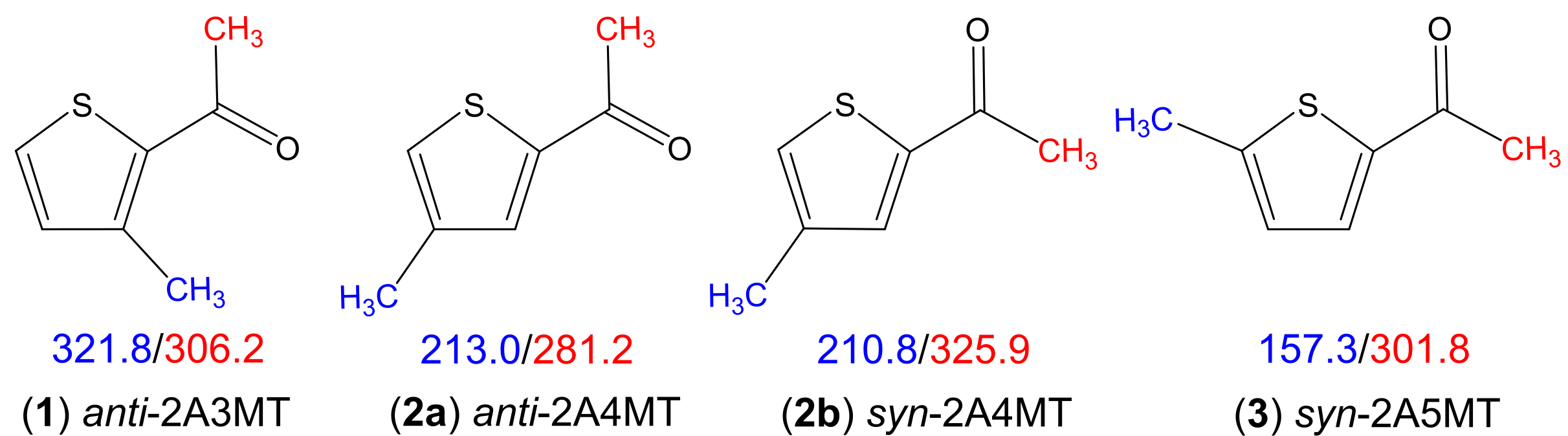
Figure 1: Experimentally observed conformers of the three acetylmethylthiophene isomers. The torsional barriers of the acetyl methyl (red) and ring methyl (blue) groups are given in cm-1.